- Silicon Number Of Electrons In Outer Shell
- Silicon Number Of Electrons Gained Or Lost
- Silicon Number Of Electron Shells
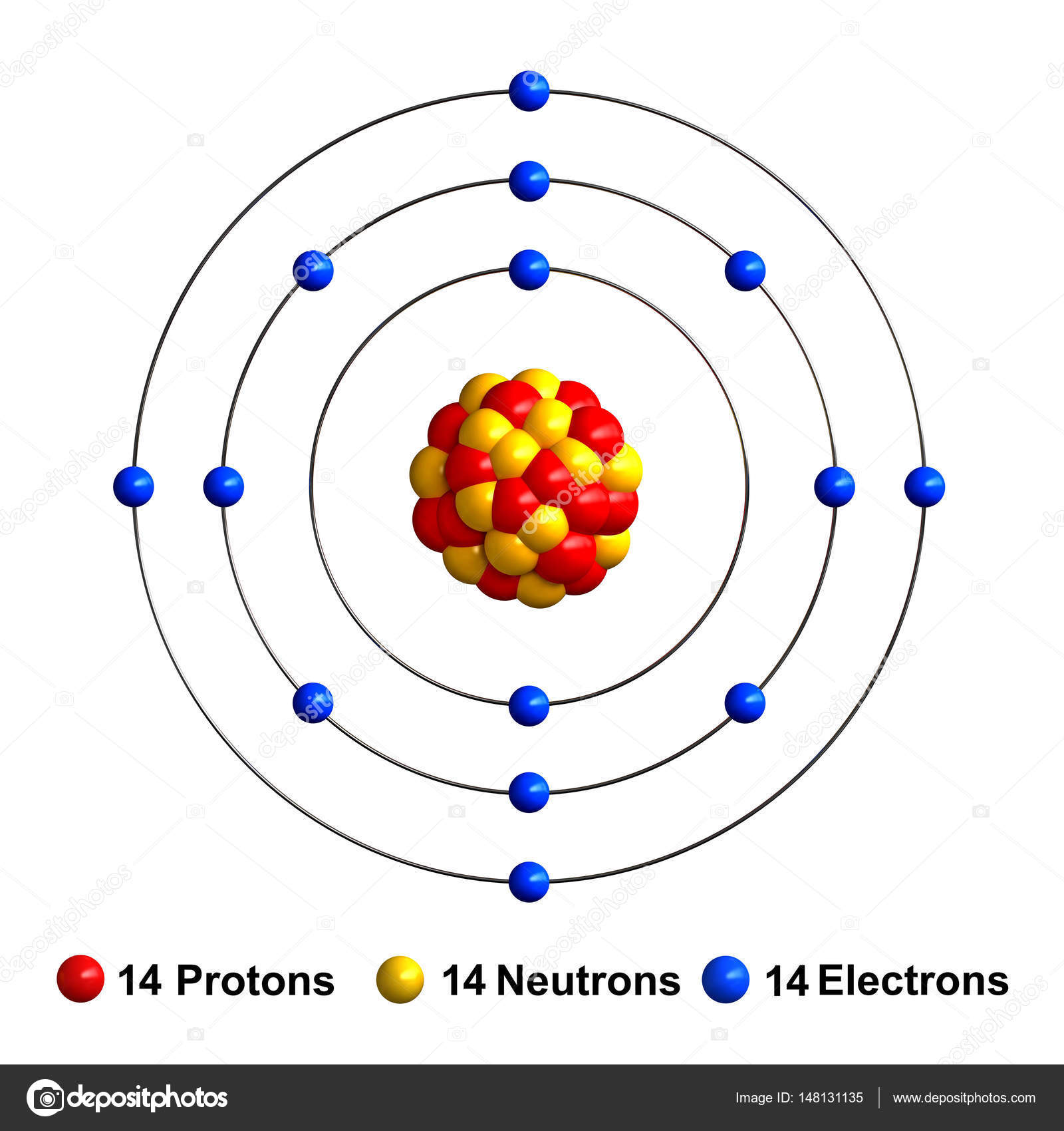
Silicon is an element with atomic number 14 which means it has 14 protons and 14 electrons. Its has isotopes 28, 29, 30, 31 and 32; to arrive at the number of neutrons for each isotope subtract 14 (the number of protons). The most common isotope is 28, with 14 neutrons. Name: Silicon Symbol: Si Atomic Number: 14 Atomic Mass: 28.0855 amu Melting Point: 1410.0 °C (1683.15 K, 2570.0 °F) Boiling Point: 2355.0 °C (2628.15 K, 4271.0 °F) Number of Protons/Electrons: 14 Number of Neutrons: 14 Classification: Metalloid Crystal Structure: Cubic Density @ 293 K: 2.329 g/cm 3 Color: grey Atomic Structure. A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. Periodic table is arranged by atomic number, number of protons in the nucleus which is same as number of electrons. The atomic number increases from left to right. Periodic table starts at top left ( Atomic number 1) and ends at bottom right (atomic number 118). Therefore you can directly look for atomic number 14 to find Silicon on periodic table.
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, and lead are below it. It is relatively unreactive.
How many valence electrons does a neutral silicon atom have?
1 Answer
You can use the position of silicon on the periodic table, or its ground state electron configuration to determine the number of valence electrons.
Explanation:
The element Silicon (Si), is in group 14/IVA on the periodic table. All of the elements in this group have four valence electrons.
Silicon Number Of Electrons In Outer Shell
A neutral silicon atom has equal numbers of protons and electrons. The number of protons is its atomic number, which is 14. So the number of electrons is 14. With this information, we can write the ground state (lowest energy) electron configuration for silicon as
Valence electrons occupy the highest energy s and p sublevels in the representative elements. So you can see by the electron configuration, that silicon has two 3s electrons and two 3p electrons, which gives a total of four valence electrons.
Silicon Number Of Electrons Gained Or Lost
So you can determine the number of valence electrons for silicon by its position on the periodic table, or by writing its ground state electron configuration.
Silicon Number Of Electron Shells
Related questions
